- cross-posted to:
- [email protected]
- cross-posted to:
- [email protected]
cross-posted from: https://lemm.ee/post/42738519
I really like the safety aspect of this, but 72% capacity after 300 cycles seems low. What’s a use case scenario where this is preferable over lipo batteries?
Much more stable chemistry. In stationary applications, like UPS systems and off grid electrical systems, lead acid is still the standard, due to having stable chemistry, very unlikely to catch fire, and a cost to capacity ratio that is still very good.
The degradation seems pretty bad, but if it’s stable from 300 cycles onwards, you could take 75% as the actual capacity of the battery.
Boats, planes, drones, phones, bikes… Anywhere that you can maximize storage cell capacity in odd shaped volumes and spaces/designs. It’s great.
Dildos
Why do you think they’re called D batteries? 😏
👆
Having looked at comparative data, it’s not really out of the norm…
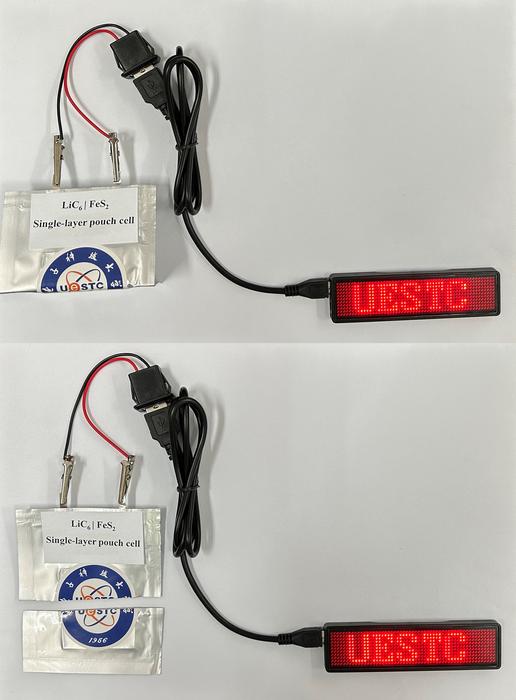
After 300 cycles, a lithium carbide iron disulfide pouch cell retained 72.0% capacity
Put that on a phone and the battery will degrade almost 30% in one year… seems a lot tbh.
As a point of reference, Google says that somewhere between 500-2000 cycles you can expect a regular lithium battery to degrade to 80%. So this is worse, but in the ballpark. Seems reasonable for a research prototype to be a little worse than a commercial product that’s had years to become highly optimized.
But depending on cost, in my hopeful optimistic universe, that could mean bringing back replaceable batteries.
But, do they cause a runaway thermal reaction if pierced?
I demand spicy pillows, not mild ones
One of the fabricated battery pouch cells was even able to work after being folded and cut off. “That proves its high safety for practical application,” the researchers emphasized.
If you can cut it in half and it still works I doubt piercing it will do much.
Oh, oh god. I know exactly how I reasoned that.
Slashing damage is different than piercing damage in the games I play. For whatever reason I ignored the context.
RPG ahh battery
it is so interesting to learn just how far behind articles like this are
I bet if you cut it vertically the lights will go out.
But does it make a boom when Mossad needs it?
Big if true
deleted by creator