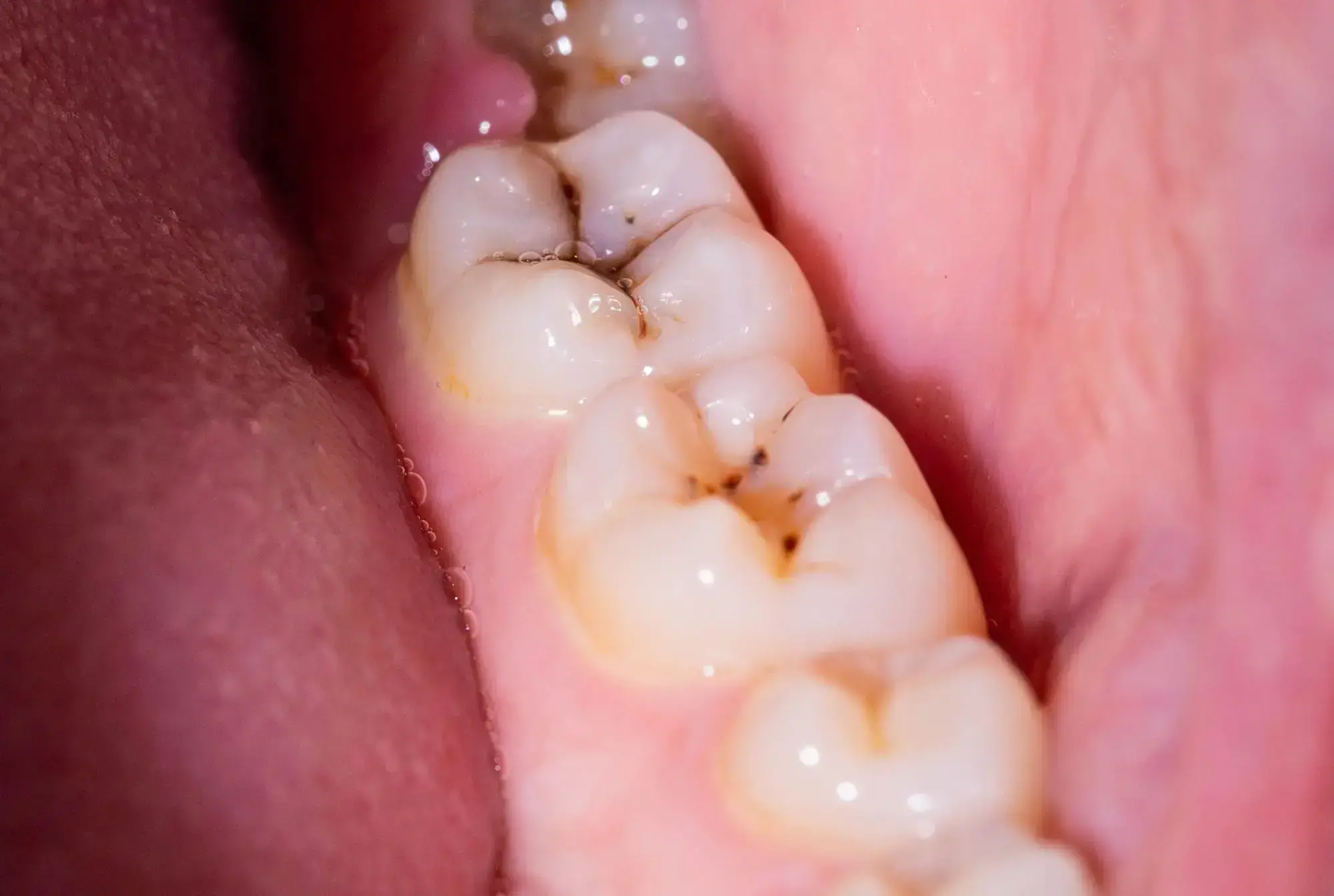
Scientists have discovered that the molecule DIM reduces biofilms causing dental plaque by 90%. Its addition to toothpaste and mouthwash could revolutionize dental hygiene. 3,3′-Diindolylmethane (DIM) decreased the Streptococcus mutans biofilm, a leading contributor to plaque and cavities, by 90%.
A significant portion of the global population experiences persistent issues with dental plaque and cavities or will face them at some time. While toothpaste, mouthwash, and routine dental visits help in prevention, there’s always room for improvement.
Researchers from Ben-Gurion University of the Negev, in collaboration with teams from Sichuan University and the National University of Singapore, have identified that 3,3′-Diindolylmethane (DIM) – a naturally occurring molecule also referred to as bisindole – can reduce biofilms responsible for plaque and cavities by a remarkable 90%.


I’ve checked the molecule in question, it’s that sort of stuff that even amateurs could make in a backyard lab, from indole and dichloromethane (use AlCl₃ as catalyst). So if the effects are real and there aren’t too big counter-effects, this will spread like wildfire.
I hope they don’t. Dichloromethane ain’t nothing to mess with. Wikipedia page section on its toxicity
That’s the cool part about chemistry, when used in this synthesis the dichloromethane becomes a whole new (presumably safe, but we’ll see what further testing turns up) molecule. Sodium is explosive and Chlorine is highly corrosive, but combine them and you get regular old table salt. Just because a reagent is dangerous doesn’t mean the products it creates will be.
Edit: I just did a little research and it looks like 3,3′-Diindolylmethane (DIM) is safe for human consumption. It’s already sold as a dietary supplement. It’s marketed to help with metabolism of estrogens but we all know how “trustworthy” the dietary supplement market is.
I don’t think they were implying that DIM is unsafe, but rather saying that they hope people don’t try to handle the dangerous reagent in their backyard.
I understand that. My concern was with amateur chemists mucking around with the dangerous reagent in their backyards. Imagine them ventilating their lab and killing the neighbours dog, things like that. Or not disposing of wastes appropriately.
By “even amateurs could make” I wanted to highlight how relatively simple of a molecule it is, not that they should. The impact is mostly industrial, those things that “even amateurs could make” tend to be really cheap.
With that in mind CH₂Cl₂ isn’t that big of a deal. It’s a reagent and it needs to be treated with respect and care; just like any other reagent. The worst mistakes don’t even happen with those dangerous chemicals, it’s with the ones that people think that are safe because they get sloppy with them. (Calcium carbide, glacial acetic acid, hydrogen peroxide, this sort of stuff.)
I changed and highlighted a word that I have concerns about. Keeping in mind, an amateur would have to make sure they correctly purify the end product, and make sure dichloromethane isn’t in the end product.
[DISCLAIMER: I’ll emphasise for the sake of clueless readers that Nobody should this at home. I’m talking on abstract grounds, I just wanted to highlight that DIM is easy to produce thus likely cheap. Plus another poster highlighted that the “magic” product is already industrially produced. OK? Seriously, backyard organics is not like backyard electrolysis, there are 100 ways to die out of it.]
Leftover DCM in the end product is the least concern. Purification is basically “add diluted acid”, keep the aqueous layer, discard/reuse the solvent layer. You could also boil it off to be extra sure, after crystallisation
The actual danger are the vapours, and mostly towards the amateur, not his guinea pig using this stuff. Carcinogenic, volatile, and flammable - if you try this on a Bunsen burner or a kitchen stove you’re risking some explosion, specially if you don’t ventilate this well. Amateurs have that tendency to think “this doesn’t drip on my hand, it’s invisible, so might as well not care that much about it”, and then they get wrecked.
It’s also the main component of some plastic model glues.
Looks like it’s being already sold for other uses.
That said this is concerning:
It could mean that the effect wasn’t really due to DIM or that dosing has to be pretty accurate to have the desired effect.
Yeah, this requirement of a very specific dosage is weird. Usually higher dosage means stronger effect, so it might be something else instead. That’s a shame.
Care to elaborate for the smooth brained?
It means that it’s relatively easy to turn a bunch of cheap ingredients into the molecule in question. So if the molecule works and doesn’t cause you harm, you can pretty much expect toothpaste manufacturers to include it in their toothpastes. That’s good because toothpastes will be better getting rid of plaque and preventing cavities.
My teeth are weaker genetically, so I have to be very firm in my dental habits. If this happens, I am going to be so, so happy.
Queue the “1 of out 10 dentists endorse this product.” messaging.
I was hoping more for a to do list for my backyard, so I’ll have the molecule myself and not be beholden to Big Toothpaste.
I’m handy, I got all kinds of tech skills, but I’ve only dabbled in chemistry. I’m not uncapable, just green and rough around the edges. I’ve done a bunch of stuff in the past, like distillations, making sodium silicate (to seal up a forge), anodizing and electroplating, or bleach thru electrolysis. I’ve also used electricity to separate and collect gases. Hell I even took a fridge apart to use it’s old compressor to compress those gasses into different tanks - all in an effort to just not have to buy CO2, nitrogen or argon. Welding with hydrogen is triiiiicky, acetylene is much nicer. I even tried cold welding in a vacuum chamber, lol, but I need a better vacuum pump, probably a rotary one. I’ve made a bunch of different kinds of batteries and super capacitors.
I’ve thought about using calcium carbide to create my own acetylene, cuz I’ve got all the equipment I would need to capture and compress it, but y’know, I like living, and acetylene isn’t THAT expensive. Risk≠reward.
So I guess that’s my limit.
I do not recommend you to do this in your backyard. And if doing this, just as a curiosity, not as a reliable production method; this is NurdRage tier, not “make bleach at home and avoid Big Tech” tier. That said, on a theoretical level, the synthesis would be simply
You’ll probably get a 10% of junk compounds where the methyl attaches itself to the wrong carbon of the indole. Those could be separated by careful crystallisation.
As long as you take the proper security procedures, this is fairly safe. One of my uncles work with car soldering, and he produces acetylene at home.
Kind of hypocrite of me to say that, given that I have a scar on one of my arms from one of those reactions, but to be fair most people aren’t as stupid as a 14yo mixing calcium carbide and water inside a glass bottle, and closing it. (It was 20 years ago. My family still mentions this.)
deleted by creator